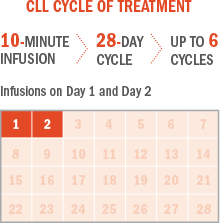
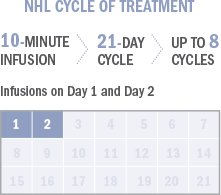
Approved Use:
BENDEKA is indicated for the treatment of patients with
· Chronic lymphocytic leukemia (CLL). Efficacy relative to first-line therapies other than chlorambucil has not been established.
· Indolent B-cell non-Hodgkin lymphoma (NHL) that has progressed during or within 6 months of treatment with rituximab or a rituximab-containing regimen.
Important Safety Information
BENDEKA is not right for everyone, including patients with a known allergic response to bendamustine, polyethylene glycol 400, propylene glycol, or monothioglycerol.
BENDEKA may cause serious side effects including: low blood cell counts, infections or recurrence of infections, unexpected responses to BENDEKA when placed in your blood, sudden and severe allergic responses, kidney failure due to fast breakdown of cancer cells, other cancers, and leaking of BENDEKA out of your vein and into your surrounding skin. Some of these side effects, such as low blood counts, infections, liver injury, and severe allergic skin responses (when bendamustine HCl was given alone and in combination with other anticancer medications or allopurinol), have caused death.
Tell your doctor if you have any side effects including:
-Signs of allergic reactions including; rash, facial swelling, or difficulty breathing during or soon after your infusion with BENDEKA injection.
-Signs of infection including; shortness of breath, significant fatigue, bleeding, bruising, fever, or other signs of infection and or any suspicious skin changes.
-Confusion, memory loss, trouble thinking, difficulty talking or walking, vision loss or other neurological or cognitive symptoms.
-Nausea, vomiting, diarrhea, loss of appetite, or a yellow skin tone.
Some serious side effects may require changes in therapy, such as lowering the amount of BENDEKA given, stopping the use of BENDEKA, or waiting longer than expected between doses of BENDEKA.
BENDEKA can cause fetal harm if taken while pregnant. If you are able to become pregnant, your healthcare provider will do a pregnancy test before starting treatment with BENDEKA. Females of reproductive potential should use effective contraception during treatment with BENDEKA and for 6 months after the last dose and for males with female partners for 3 months after the last dose. BENDEKA may also impair male fertility. Females should not breastfeed during treatment with BENDEKA and for 1
week after the last dose.
Most common side effects include: fatigue, fever, nausea, and vomiting, diarrhea, constipation, loss of appetite, cough, headache, weight loss, difficulty breathing, rash, mouth irritation, low red blood cells (oxygen-carrying cells), low platelets (blood-clotting cells), and decreased number of three different types of white blood cells (infection-fighting cells).
These are not all of the possible side effects of BENDEKA. For more information ask your healthcare provider.
You are encouraged to report side effects of prescription drugs to the FDA. Visit www.fda.gov/medwatch or call .
Please read the Full Prescribing Information.